
Deals with the frequency of molecules that collide in the correct orientation and with enough energy to initiate a reaction.Specifically relates to molecular collision.\(A\): The pre-exponential factor or frequency factor Start by setting up the initial calculation: k Ae -Ea/RT. Once you have A, you can plug it into the equation to solve for k at the temperature of 273 K. In unit of s -1(for 1 st order rate constant) or M -1s -1(for 2 nd order rate constant) With these assumptions, you can calculation the value of A at 300 K.

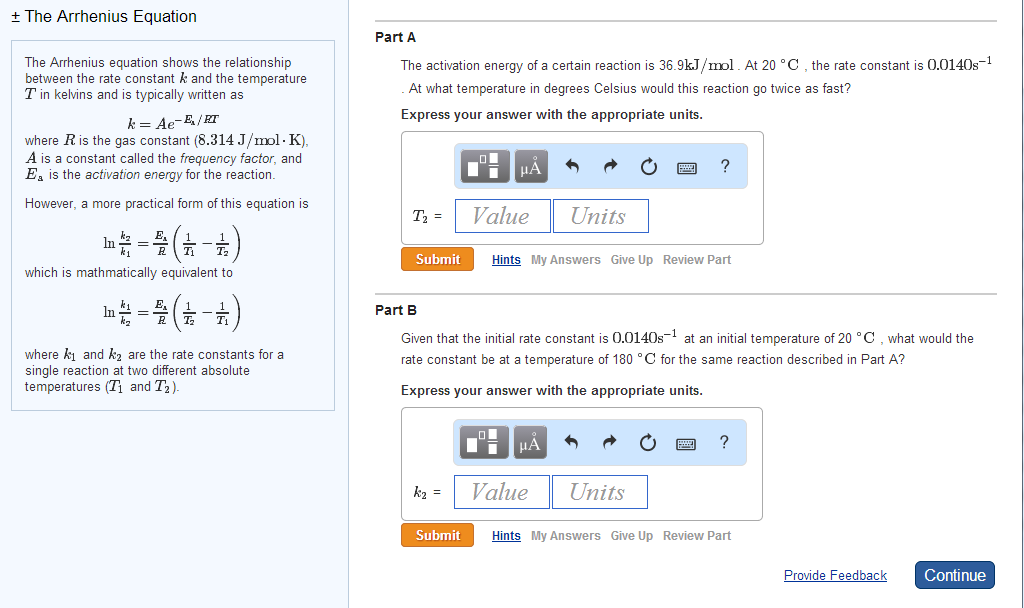
This is necessary the argument of a log function should have no units, and the number we're seeking is a ratio of quantities (rates) with the same units.\( \newcommand \nonumber\] Now we can plug in the numbers and cancel the units. To get a feel for how it works, take just the exponential term and put the negative in the exponent to work: This is reflected in the Arrhenius equation: It clearly shows that as a reaction mixture is heated, the number of particles with sufficient kinetic energy to react increases. It's worth remembering this graph and being able to sketch it yourself when you need to think about such things. And at T 3, over half of the particles have enough kinetic energy to react upon collision. In this worksheet, we will practice using the Arrhenius equation to calculate reaction activation energies and frequency (preexponential) factors Chem1305 &1306 Solutions 2 where k is the rate constant of the reaction (s 1), A is the preexponential factor (s 1), which is often called frequency factor, E is the activation energy (J mol. Transcribed image text: Use the Arrhenius Equation (Gilbert Section 13.4) to calculate the activation energy Ea for this reaction using the Experiment No. At T 2 a small fraction of all particles have speeds exceeding the activation energy, therefore some reactions can proceed. Notice that at the lowest temperature ( T 1) shown, no particles have enough energy to react - there is not enough heat in the system. The activation energy of a hypothetical reaction is shown as a dashed vertical line. Notice that the process of conversion of reactants to products proceeds through some sort of intermediate phase, called the transition state, which we usually designate with the "double dagger" symbol, ‡. The plot below shows a reaction profile for a typical exothermic (gives off heat) reaction. For the rest of the time the flame is burning, the energy normally released by the (exothermic) reaction is sufficient to activate all further combustion reactions. That small amount of energy is necessary for starting the spontaneous, self-sustaining reaction that then begins. You can let the gas out of the burner, but it won't ignite unless you provide some small spark with an igniter. A calculator is provided below to easily explore difference test scenarios. Four variables are used in calculating the accelerated aging test duration. Think about burning methane or propane gas in a Bunsen or Tirrill burner in the chemistry lab. Accelerated Aging calculation is based on Arrhenius’ equation which simply states that a 10☌ increase in temperature doubles the rate of chemical reaction. All (except for a special few) chemical reactions have an activation barrier that must be overcome before the reaction can proceed. Let's work through the elements of Arrhenius' equation.


 0 kommentar(er)
0 kommentar(er)
